- Mole is the generally accepted SI unit for measuring the amount of substance
- Mole is abbreviated to mol
- Mole has the symbol n
Defination mole in terms of C-12
One mole is the amount of substance that contains the same number of particles as the number of atoms in exactly 12.00g of C-12.
12 g of carbon-12 atoms = 6.02 x 1023 carbon atoms
6.022 x 1023 is called the Avogadro number or Avogadro constant
Avogadro's number is equal to 602,214,199,000,000,000,000,000 or more simply, 6.02214199 × 10 23
NA is the symbol used to represent the Avogadro Number
Defination mole in terms of Avogadro’s constant, NAOne mole is the amount of substance that contains Avogadro’s constant, NA of particles.
1 mole of "ANYTHING" contains 6.022 x 1023 entities.
1 mole of donuts contains 6.02 x 1023 donuts
1 mole of H2O contains 6.02 x 1023 molecules
1 mole of nails contains 6.02 x 1023 nails
1 mole of Fe contains 6.02 x 1023 atoms
1 mole of cats contains 6.02 x 1023 catss
1 mole of electrons contains 6.02 x 1023 electrons
1 mole of electrons contains 6.02 x 1023 electrons
1 MOLE = Molar Mass in gram ( Ar @ Mr )
1 MOLE = Molar Volume gas ( S.T.P @ R.T.P )
= 6.023x1023 chlorine atoms
= 35.5 g Cl
1.0 mol of chlorine molecules ( Cl2 )
= 6.023x1023 chlorine molecules
= 2(35.5) g
= 71.0 g Cl2
= 6.023x1023 x 2 chlorine atoms
1.0 mol of calcium bromide
= 6.023x1023 units of CaBr2
= 200 g CaBr2
= 6.023x1023 calcium ions
= 6.023x1023 x 2 bromide ions
1.0 mol of ammonia, NH3 contains
1.0 mol of nitrogen atoms and
3.0 mol of hydrogen atoms.
1.0 mol of phosphorus, P4 contains
4.0 mol of phosphorus atoms.
1.0 mol of Na2SO4.10H2O contains
2.0 mol of natrium ions,
1.0 mol of sulphur atoms,
4.0 mol of oxygen atoms and
10.0 mol of water molecules.

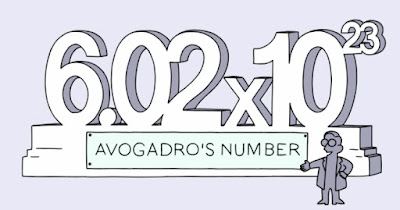



No comments:
Post a Comment