An ionic compound is chemical compound in which ions a held
together by ionic bond. Usually, the positive charged portion consists of metal
cation and negatively charged portion is an anion or polyatomic ion
CATION :
All cations are derived from metal atoms ( Na+,
Mg2+, Al3+ )except ammonium ion ; NH4+ and hydronium ion, H3O+
Many metals (particularly transition elements) can form
more than one ion, each with particular charge . Example : Fe2+ : iron(II), Fe3+ : iron(III)
ANION
Name of anion takes the root of the nonmetal and add
suffix “–ide”
Examples
are oxide (O2-),
sulfide (S2-),
fluoride (F-),
chloride (Cl-),
bromide (Br-),
iodide (I-),
nitride (N3-),
hydride (H-)
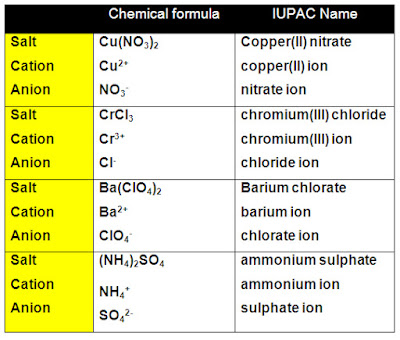
To name an ionic compound you simply need to find the names of the cation and anion present in the compound.
- Identify the cation:
- The cation is always written first in the formula for an ionic compound.
- Identify the anion:
- Cover up the cation. Everything that is leftover will be the anion.
- Write the name of the ionic compound by writing the name of the cation followed by the name of the anion
Example 1: Write the correct name for the compound whose empirical formula is Na2SO4.
- Na2SO4 is an ionic compound because it contains a metal (Na) and two non-metals (S and O).
- Identify the cation:
- The cation will be the first element written: Na2SO4
- sodium ion
- Identify the anion:
- The anion will be everything leftover once the cation has been identified: Na2SO4
- sulfate ion
- The correct name for Na2SO4 is sodium sulfate.
Example 2: Write the correct name for the compound whose empirical formula is (NH4)2CO3.
- Even though this compound is composed of only non-metals, it is still an ionic compound. (It starts with NH4.)
- Identify the cation:
- The cation will always be the first ion written.
- This compound contains the only common polyatomic cation. (NH4)2CO3
- ammonium ion
- This compound contains the only common polyatomic cation. (NH4)2CO3
- Identify the anion:
- The anion will be everything leftover once the cation has been identified: (NH4)2CO3
- carbonate ion
- The correct name for this compound is ammonium carbonate.
Example 3: Write the correct name for the compound whose empirical formula is SnO2.
- This compound is an ionic compound. It contains a metal (Sn) and a non-metal (O).
- Identify the cation:
- The cation is always written first in the formula.
- SnO2
- Tin can form two different cations Sn2+ and Sn4+). You must correctly identify its charge.
- The charges of the cation and the anions must exactly balance out.
- Since there are two anions and each one has a 2- charge, there is a total negative charge of 4-. In order to balance out the negative charge, the single tin ion must have a 4+ charge.
- tin (IV) ion
- Identify the anion:
- The anion will be everything leftover once the cation has been identified.
- SnO2
- oxide ion
- The correct name for the compound is tin (IV) oxide.


No comments:
Post a Comment