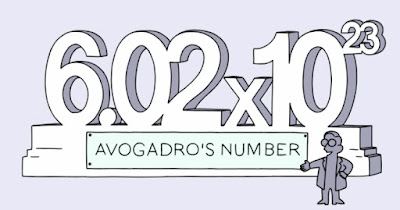Sunday, June 23, 2019
Saturday, June 22, 2019
C3 : 3.1-MOLE
In chemistry the mole is a fundamental unit in the Système International d'Unités, the SI system, and it is used to measure the amount of substance. This quantity is sometimes referred to as the chemical amount.
One mole is the amount of substance that contains Avogadro’s constant, NA of particles.
1 mole of "ANYTHING" contains 6.022 x 1023 entities.
1 MOLE = 6.02 x 1023 particles
1 MOLE = Molar Mass in gram ( Ar @ Mr )
1 MOLE = Molar Volume gas ( S.T.P @ R.T.P )
1.0 mol of chlorine atoms ( Cl )
= 6.023x1023 chlorine atoms
= 35.5 g Cl
1.0 mol of chlorine molecules ( Cl2 )
= 6.023x1023 chlorine molecules
= 2(35.5) g
= 71.0 g Cl2
= 6.023x1023 x 2 chlorine atoms
1.0 mol of calcium bromide
= 6.023x1023 units of CaBr2
= 200 g CaBr2
= 6.023x1023 calcium ions
= 6.023x1023 x 2 bromide ions
1.0 mol of ammonia, NH3 contains
1.0 mol of nitrogen atoms and
3.0 mol of hydrogen atoms.
1.0 mol of phosphorus, P4 contains
4.0 mol of phosphorus atoms.
1.0 mol of Na2SO4.10H2O contains
2.0 mol of natrium ions,
1.0 mol of sulphur atoms,
4.0 mol of oxygen atoms and
10.0 mol of water molecules.
- Mole is the generally accepted SI unit for measuring the amount of substance
- Mole is abbreviated to mol
- Mole has the symbol n
Defination mole in terms of C-12
One mole is the amount of substance that contains the same number of particles as the number of atoms in exactly 12.00g of C-12.
12 g of carbon-12 atoms = 6.02 x 1023 carbon atoms
6.022 x 1023 is called the Avogadro number or Avogadro constant
Avogadro's number is equal to 602,214,199,000,000,000,000,000 or more simply, 6.02214199 × 10 23
NA is the symbol used to represent the Avogadro Number
Defination mole in terms of Avogadro’s constant, NAOne mole is the amount of substance that contains Avogadro’s constant, NA of particles.
1 mole of "ANYTHING" contains 6.022 x 1023 entities.
1 mole of donuts contains 6.02 x 1023 donuts
1 mole of H2O contains 6.02 x 1023 molecules
1 mole of nails contains 6.02 x 1023 nails
1 mole of Fe contains 6.02 x 1023 atoms
1 mole of cats contains 6.02 x 1023 catss
1 mole of electrons contains 6.02 x 1023 electrons
1 mole of electrons contains 6.02 x 1023 electrons
1 MOLE = Molar Mass in gram ( Ar @ Mr )
1 MOLE = Molar Volume gas ( S.T.P @ R.T.P )
= 6.023x1023 chlorine atoms
= 35.5 g Cl
1.0 mol of chlorine molecules ( Cl2 )
= 6.023x1023 chlorine molecules
= 2(35.5) g
= 71.0 g Cl2
= 6.023x1023 x 2 chlorine atoms
1.0 mol of calcium bromide
= 6.023x1023 units of CaBr2
= 200 g CaBr2
= 6.023x1023 calcium ions
= 6.023x1023 x 2 bromide ions
1.0 mol of ammonia, NH3 contains
1.0 mol of nitrogen atoms and
3.0 mol of hydrogen atoms.
1.0 mol of phosphorus, P4 contains
4.0 mol of phosphorus atoms.
1.0 mol of Na2SO4.10H2O contains
2.0 mol of natrium ions,
1.0 mol of sulphur atoms,
4.0 mol of oxygen atoms and
10.0 mol of water molecules.
Friday, June 21, 2019
C3 : MOLE-MASS-NO.PARTICLE CONVERSIONS
Relationship between mole, mass, Ar @ Mr and no. of particles
SOURCE: http://elementlearning.com/
Converting Mass to Moles & Moles to Mass (by using Mr @ Ar)
Converting Moles to Mass (multiply by the molar mass)
1. How many grams are in 2.4 moles of sulfur?
ans : 2.4 x 32 = 76.8 grams
2. How many grams are in 238 moles of arsenic?
ans : 238 x 75 = 17,850 grams
3. How many grams are in 11.9 moles of chromium?
ans : 11.9 x 52 = 618.8 grams
4. How many grams are in 88.1 moles of magnesium?
ans : 88.1 x 24 = 2114.4 grams
Converting Mass to Moles (divide by the molar mass)
1. How many moles are in 15 grams of lithium?
ans : 15 / 7 = 2.14 moles
2. How many moles are in 22 grams of argon?
ans : 22 / 40 = 0.55 moles
3. How many moles are in 2.3 grams of phosphorus?
ans : 2.3 / 31 = 0.074 moles
4. How many moles are in 10.5 grams of magnesium?
ans : 10.5 / 24 = 0.438 moles
5. How many moles are in 68 grams of copper (II) hydroxide, Cu(OH)2?
ans : 68 / 99 = 0.69 moles
6. How many moles are in 98.3 grams of aluminum hydroxide, Al(OH)3?
ans : 98.3 / 78 = 1.26 moles
7. How many moles are in 9.54 grams of silicon dioxide, SO2?
ans : 9.54 / 64 = 0.149 moles
Converting Number of Particles to Moles & Moles to Number of Particles
Converting Moles to No.of atoms/molecules/ions (multiply by the NA)
1. How many atoms in 3.0 moles of Ar?
ans : 3.0 x 6.02 x 1023 = 1.806 x 1024 atoms
2. How many atoms in 8.5 moles of Fe?
ans : 8.5 x 6.023 x 1023 = 5.117 x 1024 atoms
3. How many molecules in 2.0 moles of CO2?
ans : 2.0 x 6.023 x 1023 = 1.204 x 1024 molecules
ans : no.of C atom = 1.204 x 1024 x 2 =
4. How many molecules in 1.8 moles of PCl3?
ans : 1.8 x 6.023 x 1023 = 1.0836 x 1024 molecules
5. How many molecules in 2.5 mol aluminuim sulphate, Al2(SO4)3
ans : 2.5 x 6.023 x 1023 = 1.505 x 1024 molecules
6. How many molecules in 0.25 mol of scandium(III) oxide, Sc2O3.
ans : 0.25 x 6.023 x 1023 = 1.505 x 1023 molecules
Converting Mass to Moles (divide by the NA)
SOURCE: http://elementlearning.com/
Converting Mass to Moles & Moles to Mass (by using Mr @ Ar)
Converting Moles to Mass (multiply by the molar mass)
1. How many grams are in 2.4 moles of sulfur?
ans : 2.4 x 32 = 76.8 grams
2. How many grams are in 238 moles of arsenic?
ans : 238 x 75 = 17,850 grams
3. How many grams are in 11.9 moles of chromium?
ans : 11.9 x 52 = 618.8 grams
4. How many grams are in 88.1 moles of magnesium?
ans : 88.1 x 24 = 2114.4 grams
5. How many grams are in 4.5 moles of sodium fluoride,
NaF?
ans : 4.5 x 42
= 189
grams
6. How many grams are in 0.02 moles of beryllium iodide, BeI2?
ans : 0.02 x 263 =
5.26
grams
7. How many grams are in 3.3 moles of potassium sulfide, K2S?
ans : 3.3 x 110 = 363.0 grams
1. How many moles are in 15 grams of lithium?
ans : 15 / 7 = 2.14 moles
2. How many moles are in 22 grams of argon?
ans : 22 / 40 = 0.55 moles
3. How many moles are in 2.3 grams of phosphorus?
ans : 2.3 / 31 = 0.074 moles
4. How many moles are in 10.5 grams of magnesium?
ans : 10.5 / 24 = 0.438 moles
5. How many moles are in 68 grams of copper (II) hydroxide, Cu(OH)2?
ans : 68 / 99 = 0.69 moles
6. How many moles are in 98.3 grams of aluminum hydroxide, Al(OH)3?
ans : 98.3 / 78 = 1.26 moles
7. How many moles are in 9.54 grams of silicon dioxide, SO2?
ans : 9.54 / 64 = 0.149 moles
Converting Number of Particles to Moles & Moles to Number of Particles
Converting Moles to No.of atoms/molecules/ions (multiply by the NA)
1. How many atoms in 3.0 moles of Ar?
ans : 3.0 x 6.02 x 1023 = 1.806 x 1024 atoms
2. How many atoms in 8.5 moles of Fe?
ans : 8.5 x 6.023 x 1023 = 5.117 x 1024 atoms
3. How many molecules in 2.0 moles of CO2?
ans : 2.0 x 6.023 x 1023 = 1.204 x 1024 molecules
ans : no.of C atom = 1.204 x 1024 x 2 =
4. How many molecules in 1.8 moles of PCl3?
ans : 1.8 x 6.023 x 1023 = 1.0836 x 1024 molecules
5. How many molecules in 2.5 mol aluminuim sulphate, Al2(SO4)3
ans : 2.5 x 6.023 x 1023 = 1.505 x 1024 molecules
6. How many molecules in 0.25 mol of scandium(III) oxide, Sc2O3.
ans : 0.25 x 6.023 x 1023 = 1.505 x 1023 molecules
Thursday, June 20, 2019
MOLE : PRACTICE
Mole Practice Problem : 1
1.
59 g of Hydrochloric Acid =
__________________________ moles
2.
0.46 moles of Sodium Hydroxide
= _____________________g
3.
35g of Sulfuric Acid = _______________________________ mol
4.
877 g of Potassium Permanganate
= ___________________ mol
5.
3.4 x 1024 moles of Copper (II) Bromide = _________________g
6.
0.56 moles of Calcium Hydroxide = _____________________ g
7.
4.0 x 10-23 g of Magnesium = __________________________
mol
8.
49 g of Dihydrogen Monoxide = ________________________ mol
9.
6.4 g of Iron (III) Chlorate = ____________________________ mol
10.
4.5 moles of Nitric Acid = ______________________________ g
11.
2.1 x 10-10 g of Phosphorus Pentabromide =
_______________ mol
12.
9.6 moles of Nickel (II) Sulfate = _________________________ g
13.
1.15 moles of Acetic Acid = _____________________________ g
14.
65.7 g of Silver Nitrate = ______________________________ mol
15. 0.25 moles of Strontium Phosphide = ____________________ g
C2 : 2.3-Naming Ionic Compounds
An important part of dealing with chemical compounds is knowing how to refer to them. Learn to name all ionic compounds, including simple binary compounds, compounds containing transition metals and compounds containing polyatomic ions.
To name an ionic compound you simply need to find the names of the cation and anion present in the compound.
An ionic compound is chemical compound in which ions a held
together by ionic bond. Usually, the positive charged portion consists of metal
cation and negatively charged portion is an anion or polyatomic ion
CATION :
All cations are derived from metal atoms ( Na+,
Mg2+, Al3+ )except ammonium ion ; NH4+ and hydronium ion, H3O+
Many metals (particularly transition elements) can form
more than one ion, each with particular charge . Example : Fe2+ : iron(II), Fe3+ : iron(III)
ANION
Name of anion takes the root of the nonmetal and add
suffix “–ide”
Examples
are oxide (O2-),
sulfide (S2-),
fluoride (F-),
chloride (Cl-),
bromide (Br-),
iodide (I-),
nitride (N3-),
hydride (H-)
To name an ionic compound you simply need to find the names of the cation and anion present in the compound.
- Identify the cation:
- The cation is always written first in the formula for an ionic compound.
- Identify the anion:
- Cover up the cation. Everything that is leftover will be the anion.
- Write the name of the ionic compound by writing the name of the cation followed by the name of the anion
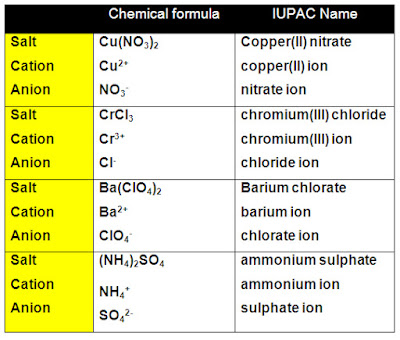
Example 1: Write the correct name for the compound whose empirical formula is Na2SO4.
- Na2SO4 is an ionic compound because it contains a metal (Na) and two non-metals (S and O).
- Identify the cation:
- The cation will be the first element written: Na2SO4
- sodium ion
- Identify the anion:
- The anion will be everything leftover once the cation has been identified: Na2SO4
- sulfate ion
- The correct name for Na2SO4 is sodium sulfate.
Example 2: Write the correct name for the compound whose empirical formula is (NH4)2CO3.
- Even though this compound is composed of only non-metals, it is still an ionic compound. (It starts with NH4.)
- Identify the cation:
- The cation will always be the first ion written.
- This compound contains the only common polyatomic cation. (NH4)2CO3
- ammonium ion
- This compound contains the only common polyatomic cation. (NH4)2CO3
- Identify the anion:
- The anion will be everything leftover once the cation has been identified: (NH4)2CO3
- carbonate ion
- The correct name for this compound is ammonium carbonate.
Example 3: Write the correct name for the compound whose empirical formula is SnO2.
- This compound is an ionic compound. It contains a metal (Sn) and a non-metal (O).
- Identify the cation:
- The cation is always written first in the formula.
- SnO2
- Tin can form two different cations Sn2+ and Sn4+). You must correctly identify its charge.
- The charges of the cation and the anions must exactly balance out.
- Since there are two anions and each one has a 2- charge, there is a total negative charge of 4-. In order to balance out the negative charge, the single tin ion must have a 4+ charge.
- tin (IV) ion
- Identify the anion:
- The anion will be everything leftover once the cation has been identified.
- SnO2
- oxide ion
- The correct name for the compound is tin (IV) oxide.
Subscribe to:
Posts (Atom)