Classification of Matters with Examples
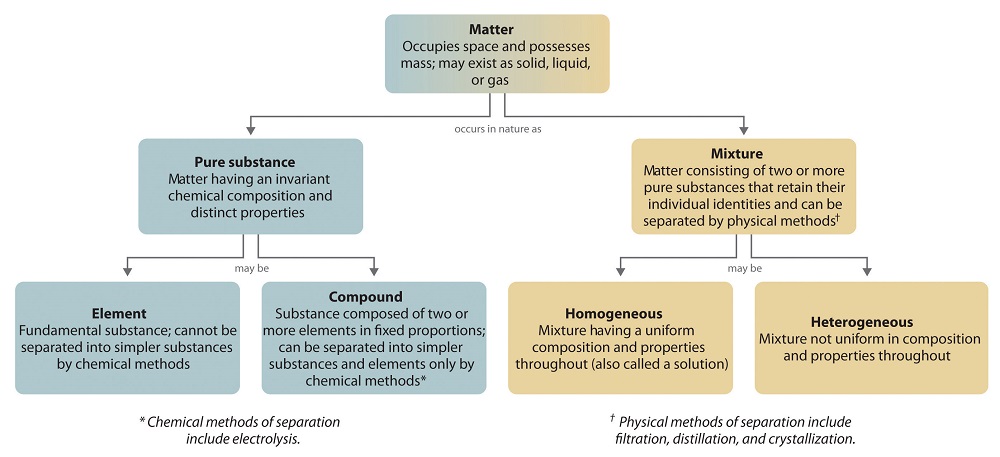
Matter is a term used for everything having mass and volume. In this unit we will deal with types of matters. Pure substance, elements, compounds, mixtures are subjects of this unit.

1) Pure Matter: Same types of atoms or molecules comprise pure matters. They have some distinguishing properties. There are two pure matters, elements and compounds. Iron, alcohol, salt are examples of pure matters.
Properties of Pure Matters:
- They are homogeneous.
- They have specific physical properties like boiling point, density or freezing point.
- Temperature during phase change is constant
Now we explain pure substances one by one.
a) Elements: Element is the simplest matter which contains one type of atom. There are 109 known element in nature. We show elements with symbols like for iron we use "Fe".
Carbon "C"
Beryllium "Be"
b) Compounds: Two or more than two elements come together in specific amounts and form new matter that we call compound. Properties of compounds are totally different from elements comprising it. We show compounds with formulas like water H2O. Ions or molecules can produce compounds.
Salt "NaCl"
Ammonia "NH3"
Iron III Oxide "Fe2O3"
Properties of Compounds:
- All compounds are pure substances
- Smallest particle of compound is molecule including different types of atoms
2) Mixture: Different two or more than two types of matter (element, molecule, compound) are mixed to get mixture. All matters forming mixture keep their original properties. They are not pure matters. We can explain mixtures under two titles, homogeneous mixtures and heterogeneous mixtures.
a) Homogeneous Mixtures: All parts of mixture show same properties in homogeneous mixtures. We can call homogeneous mixtures as solutions. Salt water, sugar water, air are examples of homogeneous mixtures.
b) Heterogeneous Mixtures: Mixtures do not show same uniformity in all parts of it. In this types of mixtures, you can see different phases of matters. Water+Sand, milk, blood, soil are some common examples of heterogeneous mixtures.
Emulsion: Heterogeneous mixture including two different liquids. For example, oil-water, gasoline-water are emulsion examples.
Suspension: Heterogeneous mixture produced by one solid and one liquid matter.Sand-water, naphthalene-water are examples of suspension.
Colloids: are heterogeneous mixture type. Solute matters are homogeneously distributed in solvent however; we can see particles of solute with naked eye or microscope in colloids but, in solutions we can not see particles with microscope. Thus; colloids are assumed to be heterogeneous mixture.
Example: Which one of the following is heterogeneous mixture?
I. Coke
II. Sea Water
III. Water+Sand
IV. Natural Gas
Coke, sea water and natural gas are homogeneous mixture but water sand is heterogeneous mixture.
Differences between Compounds and Mixtures
- Ratio between matters forming compound is constant but ratio between matters forming mixture is variable.
- Matters forming compounds loose their properties but matters forming mixtures preserve their properties.
- We can decompose compounds with chemical methods but decompose mixtures with physical methods.